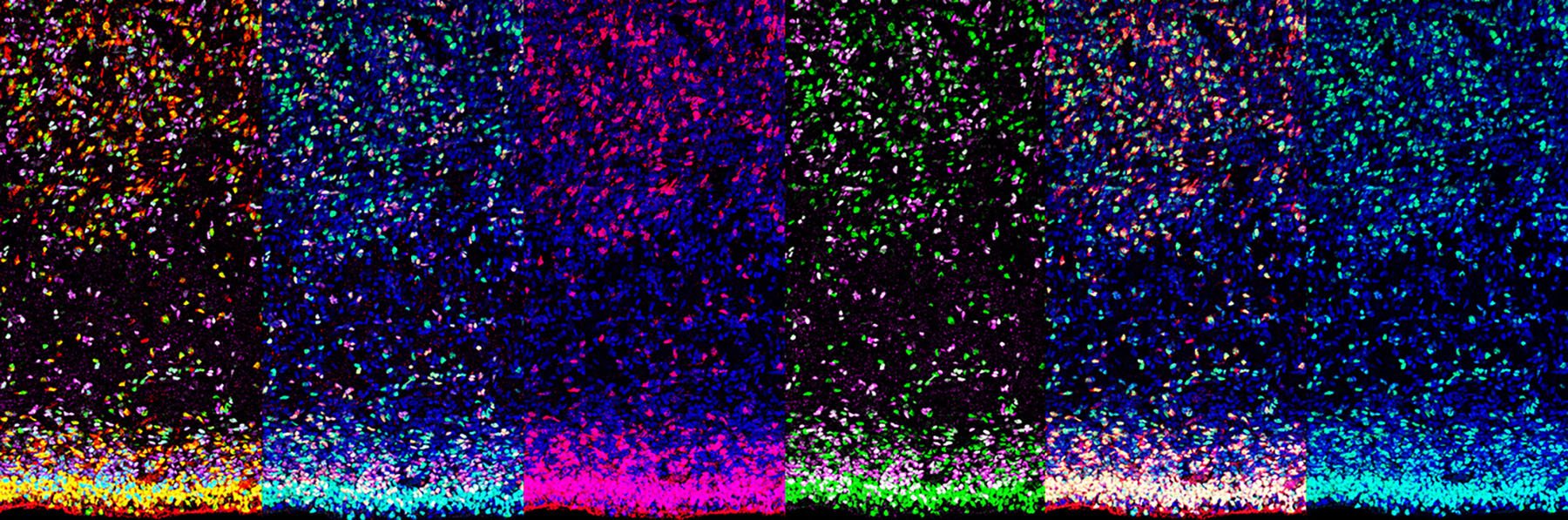
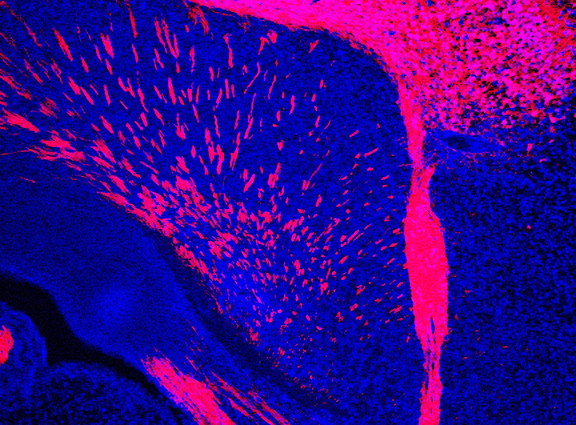
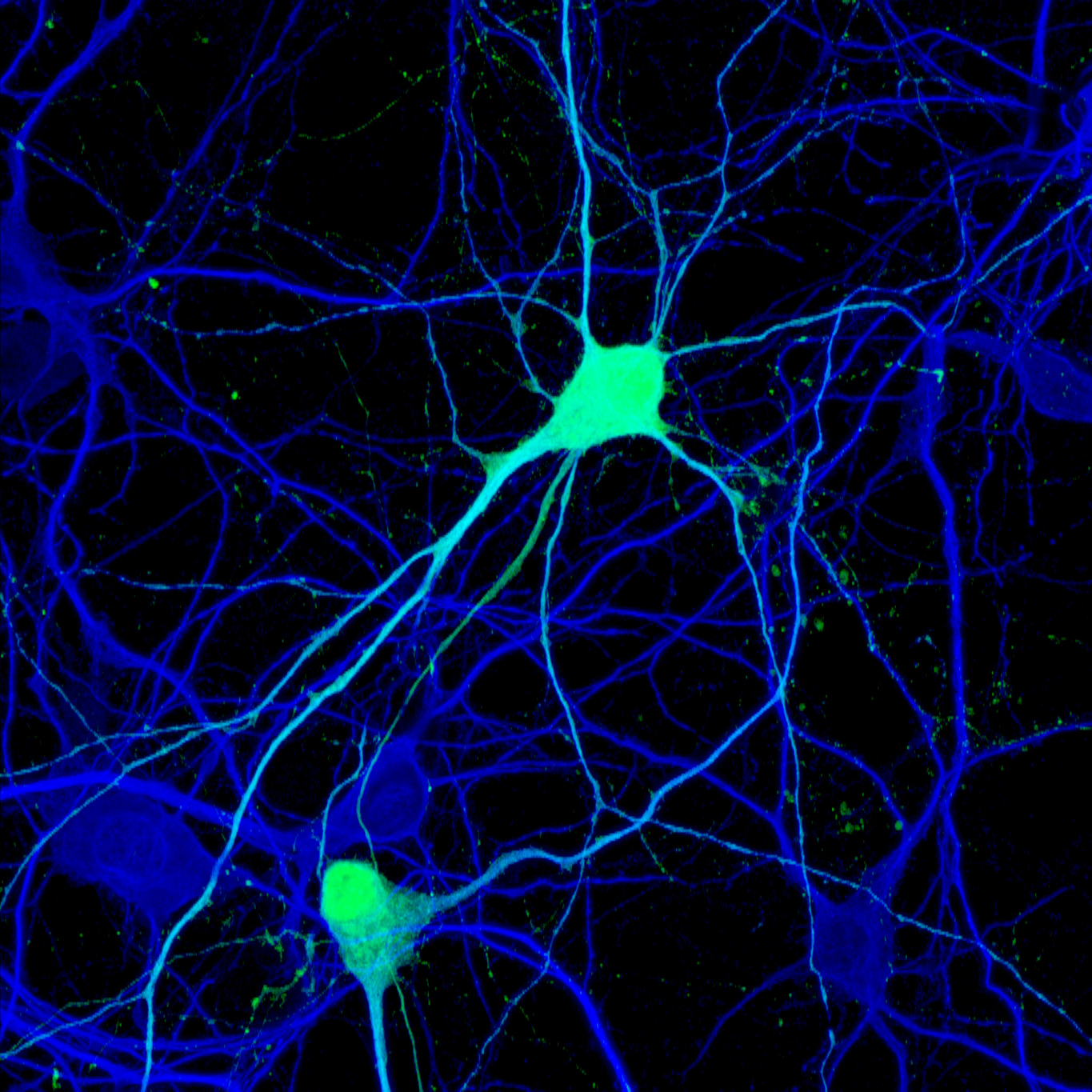
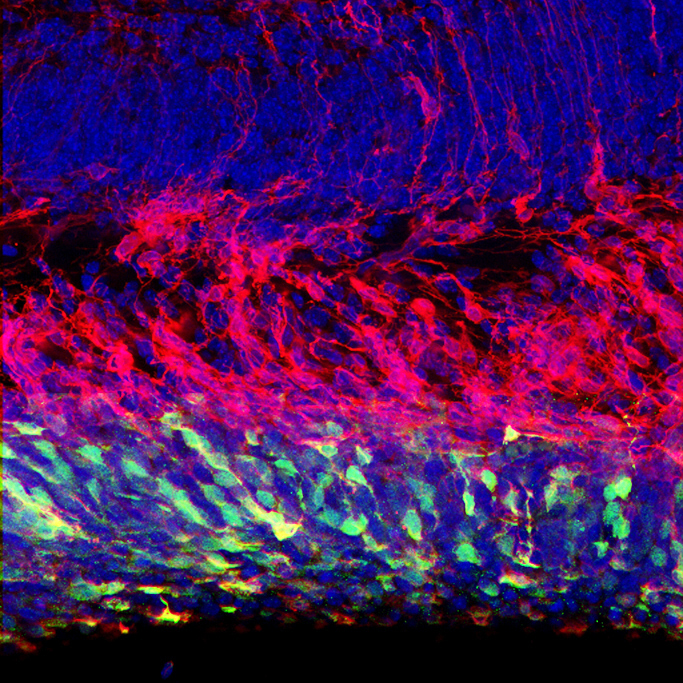
Cell diversity within an organism arises from the activation of distinct sets of genes in specific cell types. Epigenetic mechanisms, particularly those mediated by chromatin modifications, play a crucial role in enabling cells with identical genomes to express different genes. These modifications consist of chemical changes, such as DNA methylation and histone acetylation/methylation, that influence gene expression patterns. Alterations in chromatin landscapes within the developing and adult human brain are predicted to play a central role in neurological disorders. In our lab, we are investigating how genome-wide chromatin modifications regulate the specification and maintenance of cell identity in both proliferating and postmitotic cells within the mammalian cerebral cortex. Furthermore, we are exploring how chromatin modifications may influence cellular plasticity in the developing brain and throughout adulthood. Our lab has developed several mouse genetic models and a range of molecular tools to modify the epigenome of neural stem cells and cortical neurons both in vitro and in vivo. Our current research focuses on the following specific areas:
Elucidating the Role of Epigenetic Modifications in Corticogenesis
We are focused on understanding how chromatin-modifying enzymes regulate transcriptional enhancers and gene expression programs in neural stem cells, and how these processes control neurogenesis, neuronal specification, and migration within the developing cerebral cortex. Recently, we have concentrated our efforts on a specific group of histone methyltransferases that promote heterochromatin formation and play a pivotal role in various neurodevelopmental disorders, including autism. Our objective is to elucidate how genome-wide histone methylations in neural stem cells govern developmental decisions during differentiation, and how alterations in the histone methylation landscape may contribute to the onset and progression of disease.
Understanding the Mechanisms of Epigenetic Inheritance
Epigenetic inheritance is the process by which proliferating cells ensure the faithful transmission of transcriptional and chromatin states from parent to daughter cells, thereby preserving cell identity across multiple rounds of cell division. We are particularly interested in understanding how transcriptionally inactive chromatin, or heterochromatin, preserves proper gene silencing in neural stem cells during cell division. To explore this, we have developed a system that allows for the reversible removal of heterochromatin domains in primary cultures of embryonic neural stem cells. Our goal is to uncover the fundamental mechanisms of epigenetic memory and investigate their role in maintaining neural cell identity and function.
Investigating the Epigenetic Mechanisms of Developmental Cell Plasticity
During development, cells gradually restrict their potential to differentiate into multiple cell types by committing to specific fates. This process relies on the transcriptional silencing of lineage-inappropriate genes. We are investigating how heterochromatin modifications in the developing brain facilitate the silencing of alternative non-neural and neural fates, and how these modifications help maintain cell identity throughout the life of the organism. Our goal is to advance our understanding of developmental cell plasticity, which could inform the design of effective experimental strategies for cell reprogramming, with the potential for therapeutic interventions.